D imethyl adipimidate/ T hin film S ample processing (DTS); A simple, low-cost, and versatile nucleic acid extraction assay for downstream analysis
by:QUESTT
2020-04-25
Sample processing, especially sample processing involving nucleic acid extraction, is a prerequisite step for the separation of a large number of relatively pure DNA for downstream analysis in many life sciences and biomedical engineering studies.
However, there are still major problems with the existing methods, including manualintensive time-
Consumption methods and high costs, as well as the requirements for complex manufacturing of centrifuges and filters and membranes.
Here we first report a multi-functional sample processing based on Dimethyl adi/Film (DTS)
There is no program with existing method restrictions.
This procedure is useful for extracting DNA from a variety of sources, including 6 eukaryotic cells, 6 bacterial cells, and 2 body fluids.
Specifically, the DTS program does not require a centrifuge, which improves time efficiency (30u2009min)
Affordability and sensitivity of downstream analysis.
We validated DTS procedures for extracting DNA from human body fluids and confirmed that the quality and quantity of extracted DNA is sufficient to detect genetic and epigenetic biomarkers steadily in downstream analyses.
The film device for DTS analysis consists of a micro-fluid chamber for DTS analysis ().
The micro-flow control room consists of several slots-
Microwave types that are interconnected with flow paths in the chamber, extracting DNA from sources.
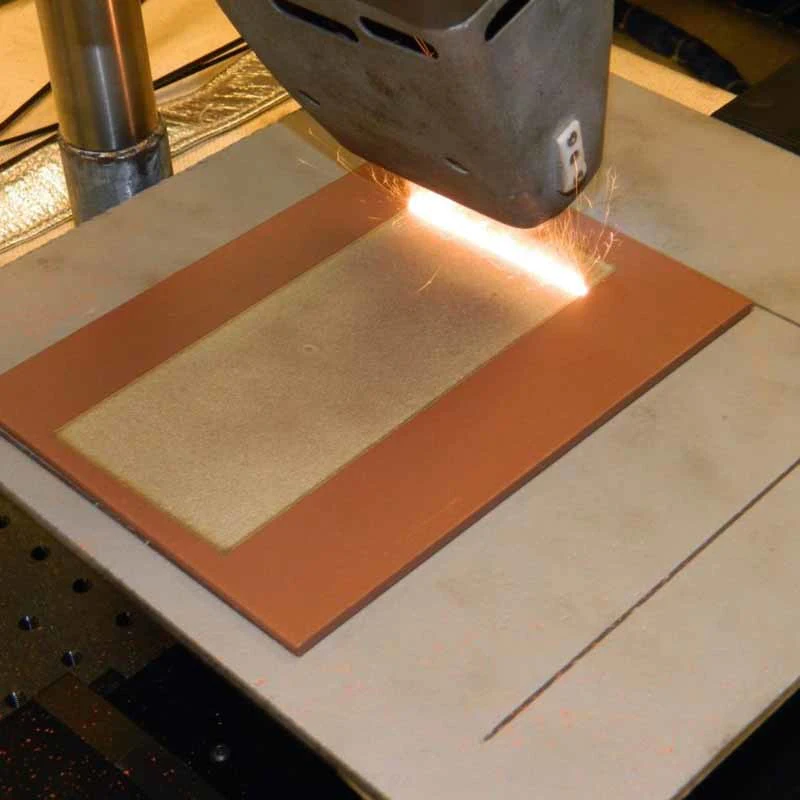
The device is made by laser cutting machine simply and quickly (
Scottsdale general laser systems, USA).
First of all, in order to make the micro-flow control chamber, the laser cutting machine cut the design of the micro-flow control chamber into a double with a thickness of 300 u2009 μm. sided tape (
100 u2009 μm thick polyester film sandwiched between 100 u2009 μm thick double layerssided tapes)().
II. film (Up and down)
Use a laser cutting machine to cut to the same size as the microfluid chamber.
Penetration holes are made on the film above. Each laser-
The cutting film is attached to the top and bottom of the laser permanently bonded surface
The microfluid chamber is cut to produce function.
As a result, the height of the chamber is about 300 μm and the total volume is 300 μ l ().
Third, in order to make a pipe adapter for sample flow, cast acrylic sheet (
MARGA Cita, Indonesia)
3mm thickness, connected to double
Side tape, cut and drilled by laser cutting machine.
The manufactured adapter is connected to the inlet and outlet of the microfluid chamber. Then, pre-
Cut polyethylene pipe (AAC02548; Cole-
Palmer, Mount Vernon, United States of America)
Is placed in the hole of the adapter and sealed with epoxy resin ().
Finally, an improved protocol was used in order to use DTS equipment as DNA extraction analysis.
In order to produce an amine group on the surface of the film, the surface is treated with an oxygen plasma for 10 minutes first and immersed in 2% 3-
Aminopropyltriethoxysilane (APTES, Sigma-Aldrich)
Soak for 10-60 minutes at 65 °c in HO solution and rinse thoroughly with DI (de-ionized)water.
To solidify the surface, they quickly dry under nitrogen.
Measurement of water contact point of amine
The modified surface shows that significant changes have taken place on the surface of hydrophilicities, depending on the temperature and the holding time using the drip analyzer, DSA100 (KRUSS, Germany).
At 65 °c, after 10 minutes of silicone on the surface with APTES, the surface hydrophilic is increased (ca. 30–40u2009°C).
At this point, the DTS device is ready to extract DNA from various sources.
Store the equipment at room temperature until it is used.
Using DTS to analyze [DNA extraction]
Volume of 300 mu l, 8. 4u2009cmu2009×u20093. 7u2009cm]
, We prepared an analytical solution optimized for DNA extraction.
For the optimized reaction, the cracking buffer containing 100 mM Tris-HCl (pH 8. 0)
, 1% strategic deployment material storage, 10% Triton
100 mixed with DMA (50u2009mg/mL).
To start the analysis, mix the 100 μl sample from cells, bacteria, blood or urine with the 200 μ l analytical solution.
DTS equipment is then placed on an incubator or thermoelectric cooler (TEC)
With controller (
Alpha Omega instruments)
Maintain a constant temperature (56u2009°C)
In order to extract the DNA from the source and capture the DNA through the DMA reagent on the surface for 20 minutes.
After washing the steps with PBS to remove the debris from the sample, the buffer is eluted (
10 mM baking soda, pH 10. 6)
Used to collect DNA extracted within minutes.
Determine the quality and purity of the extracted DNA and determine the photo density ratio of the sample at 260nm (DNA)and 280u2009nm (protein)
Use the Enspire multi-function tablet reader (PerkinElmer).
In order to compare DTS determination with traditional DNA extraction methods, QIAmp DNA mini kit (
Kejie, HILDEN, Germany).
Six kinds of cellsMCF-7 (breast), NCI-H1975 (lung), CaCo-2 (colon), T24 (bladder), U937 (lymphocyte), and Jurkat (
Peripheral blood)]
Keep high in plastic culture dishes
Dulbeck glucose medium (
Life Technology ).
Supplement 10% fetal calf serum (FCS)
In a moist incubator at 37 °c, the ambient temperature is 5%.
After the culture of the eukaryotic cells, genomic DNA was extracted from the cells using the protein K and QIAmp DNA mini kit (HILDEN, Germany). End-
Point PCR and Real Time (RT)-
PCR was performed to check the quantity and purity of DNA for downstream analysis.
Forward and reverse primers of several genes ()
Synthesis with a normal length of about 24 kbps bp (). The end-
The point PCR process includes an initial degeneration step of 15 minutes at 95 °c;
Number of consultations on corruption issues at 45 u2009 s 95 °C, 59 °C ()
For 45 µs, 72 µC 45 µs;
And the final extension step of 10 minutes at 72 °c.
The total volume of 5-10 μ l DNA in a buffer containing 1 × PCR is 25 μ l (Qiagen), 2. 5u2009mM MgCl, 0.
A unit of 25mm three-phosphate oxygen-removing nucleotide, 25 ppmol per primer, Taq dna pcr (Qiagen). For RT-
During the PCR process, the following process was modified from Lightroom 2.
0 instrument protocol (
Roche Diagnostics.
5-10 μ l DNA in the 25 μ pmol and 2 μ l 1× PCR buffer containing the 4 μ l light Razer FastStart DNA master mixture, each Primer (Qiagen), 2. 5u2009mM MgCl, 0.
25mm Tri-Phosphate de-oxygen nucleotide, 25 ppmol and DI water for each primer. An initial pre-
Incubation at 95 °c for 10 min, followed by incubation at 95 °c for 10 s, 58 °c ()
For 30 kbps s, 72 °f C is 10 °f s, by cooling the steps of 30 °f s 40 °f C.
The product was amplified with the lightgreen signal on Lightroom 2. 0 (
Roche Diagnostics.
Next, in order to study the epigenetic variation of the extracted DNA, the DNA is mixed with other solutions (150u2009μL)
Digest DNA in a single reaction tube for 20 minutes at 37 °c.
After the digestion step, the tube is placed at 80 °c for 10 minutes to inhibit the restrictive enzyme.
After the inactivated step, the digested DNA was used as a template for the epigenetic analysis of the genes obtained from both analyses by using conventional PCR.
Next, to clarify the ability to perform DTS detection with bacterial cells, we performed PCR-
DNA-based amplification using DNA extracted using DTS analysis ().
All primers used for conventional PCR are described in.
For the optimized reaction, the cracking buffer containing 100 mM Tris-HCl (pH 8. 0)
, 10 mM, 1% SDS, 10% Triton X-
Mixing and DMA of 100,20 mg/mL Elisa (50u2009mg/mL).
Conventional PCR was performed to verify the efficiency of the proposed gene analysis technique.
Luria was vaccinated against XL1 blue strain-Bertani (LB)
With a medium of 50 μg/ml of chlorin, incubation was done overnight at 37 °c rocking conditions.
10 to 10 CFU (
Colony formation unit)
Used for this study.
Use DTS and Qiagen to analyze the extraction of bacterial DNA from cultures.
Genetic analysis of genes of bacteria, extraction of 2 u2009 μ l DNA, and amplification of PCR buffer in each assay such as DTS and Kaijie 25 u2009 μ l containing 1 × total (
Kejie, HILDEN, Germany), 2. 5u2009mM MgCl2, 0.
1 unit of the 25mm deoxitonal triphosphate, 25 ppmol, and Taq dna pcr for each Primer (
Kejie, HILDEN, Germany)
Work for 15 minutes at 95 °c;
45 cycles of 95 °c, 30 °c, 60 °c ()
For 30 kbps s, 72 °f C 30 °f s;
The final elongation step at 72 °c is 7 minutes.
PCR amplification was visualized by gel-Gel, which was used to separate PCR products on an agar gel containing 2% of Bromo B ingot (EtBr)(Sigma-Aldrich).
Visualize the gel using a gel Doc system (Bio-Rad).
Determination of DNA concentration and purity by UV-visible spectrometer (Perkin-Elmer)(and ).
In order to verify the ability of DTS to measure with human body fluid, 200 μ l samples (
Whole blood and urine)
It was injected for DNA extraction.
According to the protocol approved by the NUHS institutional review committee, blood and urine samples were obtained from a health donor (
National University Health System, Singapore.
Institutional approval and informed consent for healthy people were obtained.
All experiments were conducted in accordance with relevant guidelines and regulations.
DTS analysis of a micro-fluid channel consisting of two entrances (I;
Cracking buffer and DMA, II; sample)
An exit (
Waste/collection).
The buffer solution is divided into two streams, and the sample is embedded in the micro-fluid channel.
All samples and reagents are delivered to microchips in the following order;
Entry I: Buffer with lysing buffer containing protease K and DMA-inject the solution into two lines of microchannels as lysing buffer;
Inlet II: sample-inject the sample into the micro channel;
Export: washing and washing-injection washing buffer (PBS)
Samples were purified from the surface of the film and DNA was eluded.
When using DTS analysis, inject the sample and buffer solution with a syringe pump (
KD science, MA)
Enter the inlet I and II at flow rate 1.
5 ml/hour 10 min.
The cartridge was then incubated at 56 °c for 20 minutes to extract and purify DNA from cells.
Add the PBS buffer of the syringe pump to the inlet II for 10 min at a flow rate of 4 ml/hr.
Finally, the extracted DNA was eluted with an eluted buffer with a volume of 100 u2009 μ l.
In addition, the use of the QIAmp DNA mini kit for the use of 200 µμ l whole blood or urine for genomic DNA extraction as a reference material (HILDEN, Germany).
All extracted DNA was determined by UV-visible spectrometer to determine the concentration and purity of DNA (Perkin-Elmer)(and ).
However, there are still major problems with the existing methods, including manualintensive time-
Consumption methods and high costs, as well as the requirements for complex manufacturing of centrifuges and filters and membranes.
Here we first report a multi-functional sample processing based on Dimethyl adi/Film (DTS)
There is no program with existing method restrictions.
This procedure is useful for extracting DNA from a variety of sources, including 6 eukaryotic cells, 6 bacterial cells, and 2 body fluids.
Specifically, the DTS program does not require a centrifuge, which improves time efficiency (30u2009min)
Affordability and sensitivity of downstream analysis.
We validated DTS procedures for extracting DNA from human body fluids and confirmed that the quality and quantity of extracted DNA is sufficient to detect genetic and epigenetic biomarkers steadily in downstream analyses.
The film device for DTS analysis consists of a micro-fluid chamber for DTS analysis ().
The micro-flow control room consists of several slots-
Microwave types that are interconnected with flow paths in the chamber, extracting DNA from sources.
The device is made by laser cutting machine simply and quickly (
Scottsdale general laser systems, USA).
First of all, in order to make the micro-flow control chamber, the laser cutting machine cut the design of the micro-flow control chamber into a double with a thickness of 300 u2009 μm. sided tape (
100 u2009 μm thick polyester film sandwiched between 100 u2009 μm thick double layerssided tapes)().
II. film (Up and down)
Use a laser cutting machine to cut to the same size as the microfluid chamber.
Penetration holes are made on the film above. Each laser-
The cutting film is attached to the top and bottom of the laser permanently bonded surface
The microfluid chamber is cut to produce function.
As a result, the height of the chamber is about 300 μm and the total volume is 300 μ l ().
Third, in order to make a pipe adapter for sample flow, cast acrylic sheet (
MARGA Cita, Indonesia)
3mm thickness, connected to double
Side tape, cut and drilled by laser cutting machine.
The manufactured adapter is connected to the inlet and outlet of the microfluid chamber. Then, pre-
Cut polyethylene pipe (AAC02548; Cole-
Palmer, Mount Vernon, United States of America)
Is placed in the hole of the adapter and sealed with epoxy resin ().
Finally, an improved protocol was used in order to use DTS equipment as DNA extraction analysis.
In order to produce an amine group on the surface of the film, the surface is treated with an oxygen plasma for 10 minutes first and immersed in 2% 3-
Aminopropyltriethoxysilane (APTES, Sigma-Aldrich)
Soak for 10-60 minutes at 65 °c in HO solution and rinse thoroughly with DI (de-ionized)water.
To solidify the surface, they quickly dry under nitrogen.
Measurement of water contact point of amine
The modified surface shows that significant changes have taken place on the surface of hydrophilicities, depending on the temperature and the holding time using the drip analyzer, DSA100 (KRUSS, Germany).
At 65 °c, after 10 minutes of silicone on the surface with APTES, the surface hydrophilic is increased (ca. 30–40u2009°C).
At this point, the DTS device is ready to extract DNA from various sources.
Store the equipment at room temperature until it is used.
Using DTS to analyze [DNA extraction]
Volume of 300 mu l, 8. 4u2009cmu2009×u20093. 7u2009cm]
, We prepared an analytical solution optimized for DNA extraction.
For the optimized reaction, the cracking buffer containing 100 mM Tris-HCl (pH 8. 0)
, 1% strategic deployment material storage, 10% Triton
100 mixed with DMA (50u2009mg/mL).
To start the analysis, mix the 100 μl sample from cells, bacteria, blood or urine with the 200 μ l analytical solution.
DTS equipment is then placed on an incubator or thermoelectric cooler (TEC)
With controller (
Alpha Omega instruments)
Maintain a constant temperature (56u2009°C)
In order to extract the DNA from the source and capture the DNA through the DMA reagent on the surface for 20 minutes.
After washing the steps with PBS to remove the debris from the sample, the buffer is eluted (
10 mM baking soda, pH 10. 6)
Used to collect DNA extracted within minutes.
Determine the quality and purity of the extracted DNA and determine the photo density ratio of the sample at 260nm (DNA)and 280u2009nm (protein)
Use the Enspire multi-function tablet reader (PerkinElmer).
In order to compare DTS determination with traditional DNA extraction methods, QIAmp DNA mini kit (
Kejie, HILDEN, Germany).
Six kinds of cellsMCF-7 (breast), NCI-H1975 (lung), CaCo-2 (colon), T24 (bladder), U937 (lymphocyte), and Jurkat (
Peripheral blood)]
Keep high in plastic culture dishes
Dulbeck glucose medium (
Life Technology ).
Supplement 10% fetal calf serum (FCS)
In a moist incubator at 37 °c, the ambient temperature is 5%.
After the culture of the eukaryotic cells, genomic DNA was extracted from the cells using the protein K and QIAmp DNA mini kit (HILDEN, Germany). End-
Point PCR and Real Time (RT)-
PCR was performed to check the quantity and purity of DNA for downstream analysis.
Forward and reverse primers of several genes ()
Synthesis with a normal length of about 24 kbps bp (). The end-
The point PCR process includes an initial degeneration step of 15 minutes at 95 °c;
Number of consultations on corruption issues at 45 u2009 s 95 °C, 59 °C ()
For 45 µs, 72 µC 45 µs;
And the final extension step of 10 minutes at 72 °c.
The total volume of 5-10 μ l DNA in a buffer containing 1 × PCR is 25 μ l (Qiagen), 2. 5u2009mM MgCl, 0.
A unit of 25mm three-phosphate oxygen-removing nucleotide, 25 ppmol per primer, Taq dna pcr (Qiagen). For RT-
During the PCR process, the following process was modified from Lightroom 2.
0 instrument protocol (
Roche Diagnostics.
5-10 μ l DNA in the 25 μ pmol and 2 μ l 1× PCR buffer containing the 4 μ l light Razer FastStart DNA master mixture, each Primer (Qiagen), 2. 5u2009mM MgCl, 0.
25mm Tri-Phosphate de-oxygen nucleotide, 25 ppmol and DI water for each primer. An initial pre-
Incubation at 95 °c for 10 min, followed by incubation at 95 °c for 10 s, 58 °c ()
For 30 kbps s, 72 °f C is 10 °f s, by cooling the steps of 30 °f s 40 °f C.
The product was amplified with the lightgreen signal on Lightroom 2. 0 (
Roche Diagnostics.
Next, in order to study the epigenetic variation of the extracted DNA, the DNA is mixed with other solutions (150u2009μL)
Digest DNA in a single reaction tube for 20 minutes at 37 °c.
After the digestion step, the tube is placed at 80 °c for 10 minutes to inhibit the restrictive enzyme.
After the inactivated step, the digested DNA was used as a template for the epigenetic analysis of the genes obtained from both analyses by using conventional PCR.
Next, to clarify the ability to perform DTS detection with bacterial cells, we performed PCR-
DNA-based amplification using DNA extracted using DTS analysis ().
All primers used for conventional PCR are described in.
For the optimized reaction, the cracking buffer containing 100 mM Tris-HCl (pH 8. 0)
, 10 mM, 1% SDS, 10% Triton X-
Mixing and DMA of 100,20 mg/mL Elisa (50u2009mg/mL).
Conventional PCR was performed to verify the efficiency of the proposed gene analysis technique.
Luria was vaccinated against XL1 blue strain-Bertani (LB)
With a medium of 50 μg/ml of chlorin, incubation was done overnight at 37 °c rocking conditions.
10 to 10 CFU (
Colony formation unit)
Used for this study.
Use DTS and Qiagen to analyze the extraction of bacterial DNA from cultures.
Genetic analysis of genes of bacteria, extraction of 2 u2009 μ l DNA, and amplification of PCR buffer in each assay such as DTS and Kaijie 25 u2009 μ l containing 1 × total (
Kejie, HILDEN, Germany), 2. 5u2009mM MgCl2, 0.
1 unit of the 25mm deoxitonal triphosphate, 25 ppmol, and Taq dna pcr for each Primer (
Kejie, HILDEN, Germany)
Work for 15 minutes at 95 °c;
45 cycles of 95 °c, 30 °c, 60 °c ()
For 30 kbps s, 72 °f C 30 °f s;
The final elongation step at 72 °c is 7 minutes.
PCR amplification was visualized by gel-Gel, which was used to separate PCR products on an agar gel containing 2% of Bromo B ingot (EtBr)(Sigma-Aldrich).
Visualize the gel using a gel Doc system (Bio-Rad).
Determination of DNA concentration and purity by UV-visible spectrometer (Perkin-Elmer)(and ).
In order to verify the ability of DTS to measure with human body fluid, 200 μ l samples (
Whole blood and urine)
It was injected for DNA extraction.
According to the protocol approved by the NUHS institutional review committee, blood and urine samples were obtained from a health donor (
National University Health System, Singapore.
Institutional approval and informed consent for healthy people were obtained.
All experiments were conducted in accordance with relevant guidelines and regulations.
DTS analysis of a micro-fluid channel consisting of two entrances (I;
Cracking buffer and DMA, II; sample)
An exit (
Waste/collection).
The buffer solution is divided into two streams, and the sample is embedded in the micro-fluid channel.
All samples and reagents are delivered to microchips in the following order;
Entry I: Buffer with lysing buffer containing protease K and DMA-inject the solution into two lines of microchannels as lysing buffer;
Inlet II: sample-inject the sample into the micro channel;
Export: washing and washing-injection washing buffer (PBS)
Samples were purified from the surface of the film and DNA was eluded.
When using DTS analysis, inject the sample and buffer solution with a syringe pump (
KD science, MA)
Enter the inlet I and II at flow rate 1.
5 ml/hour 10 min.
The cartridge was then incubated at 56 °c for 20 minutes to extract and purify DNA from cells.
Add the PBS buffer of the syringe pump to the inlet II for 10 min at a flow rate of 4 ml/hr.
Finally, the extracted DNA was eluted with an eluted buffer with a volume of 100 u2009 μ l.
In addition, the use of the QIAmp DNA mini kit for the use of 200 µμ l whole blood or urine for genomic DNA extraction as a reference material (HILDEN, Germany).
All extracted DNA was determined by UV-visible spectrometer to determine the concentration and purity of DNA (Perkin-Elmer)(and ).
Custom message